Pfizer recalls millions of migraine prescription drug packs; Here’s what you need know
Pfizer is recalling more than four million packages of migraine medication.
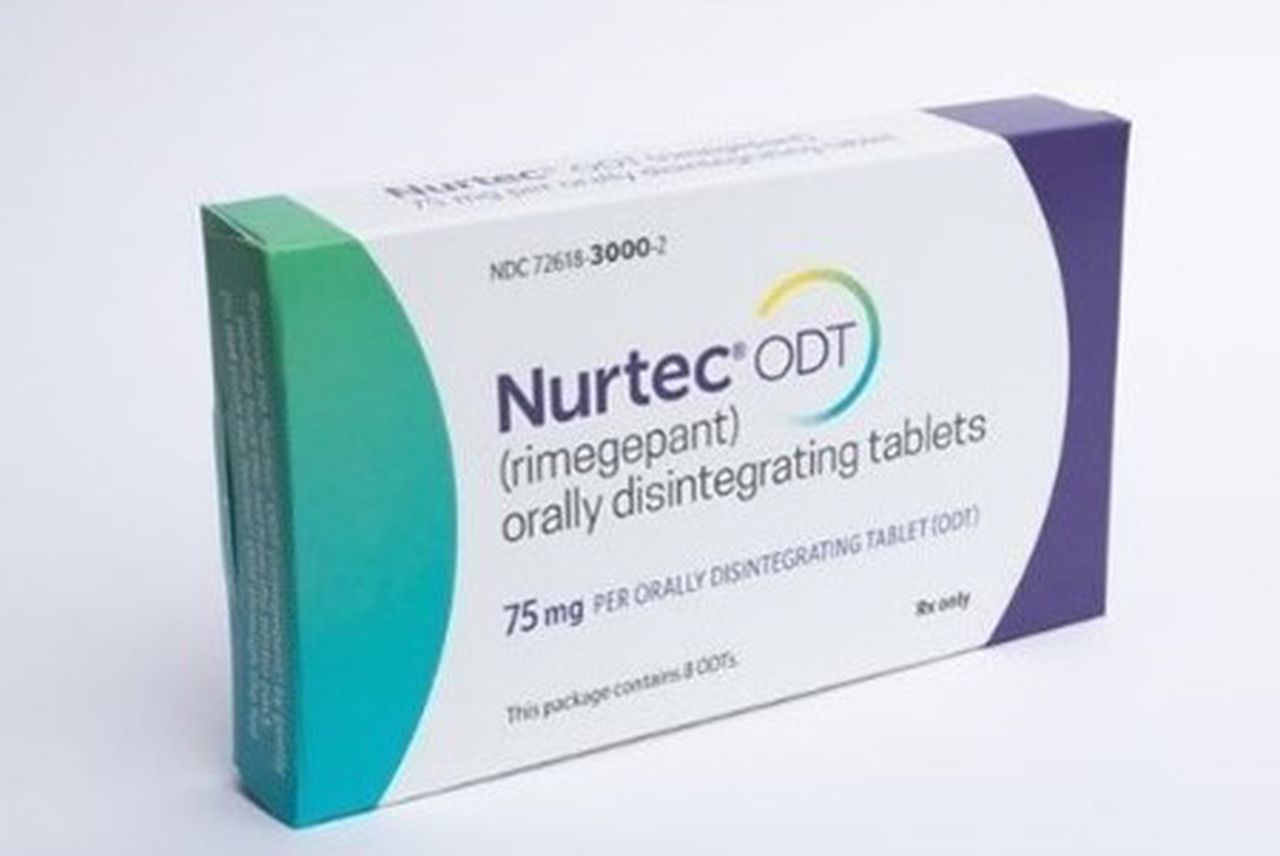
Nurtec ODT, a prescription drug, is being pulled due to a failure to meet child-resistant packaging safety requirements. The result could lead to poisoning.
The company is recalling around 4.2 million units of Nurtec ODT (rimegepant) 75mg orally disintegrating tablets, which are sold in packs of eight doses on a blister card, on Thursday, the U.S. Consumer Product Safety Commission said.
Anyone affected will be able to obtain a free child-resistant pouch to store the product, the commission said.
“The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children,” it warned.
Consumers were told to “immediately secure the recalled product out of the sight and reach of children and contact Pfizer for a free child resistant pouch to store the product.”
As of Thursday, no incidents had been reported in connection with the issue, the safety commission said.
The Associated Press contributed to this report.