Nasal spray recall: 3 types recalled over potential for âlife-threateningâ contamination
Three types of nasal spray rinses are being recalled over concerns of the possibility of “life-threatening” contamination.
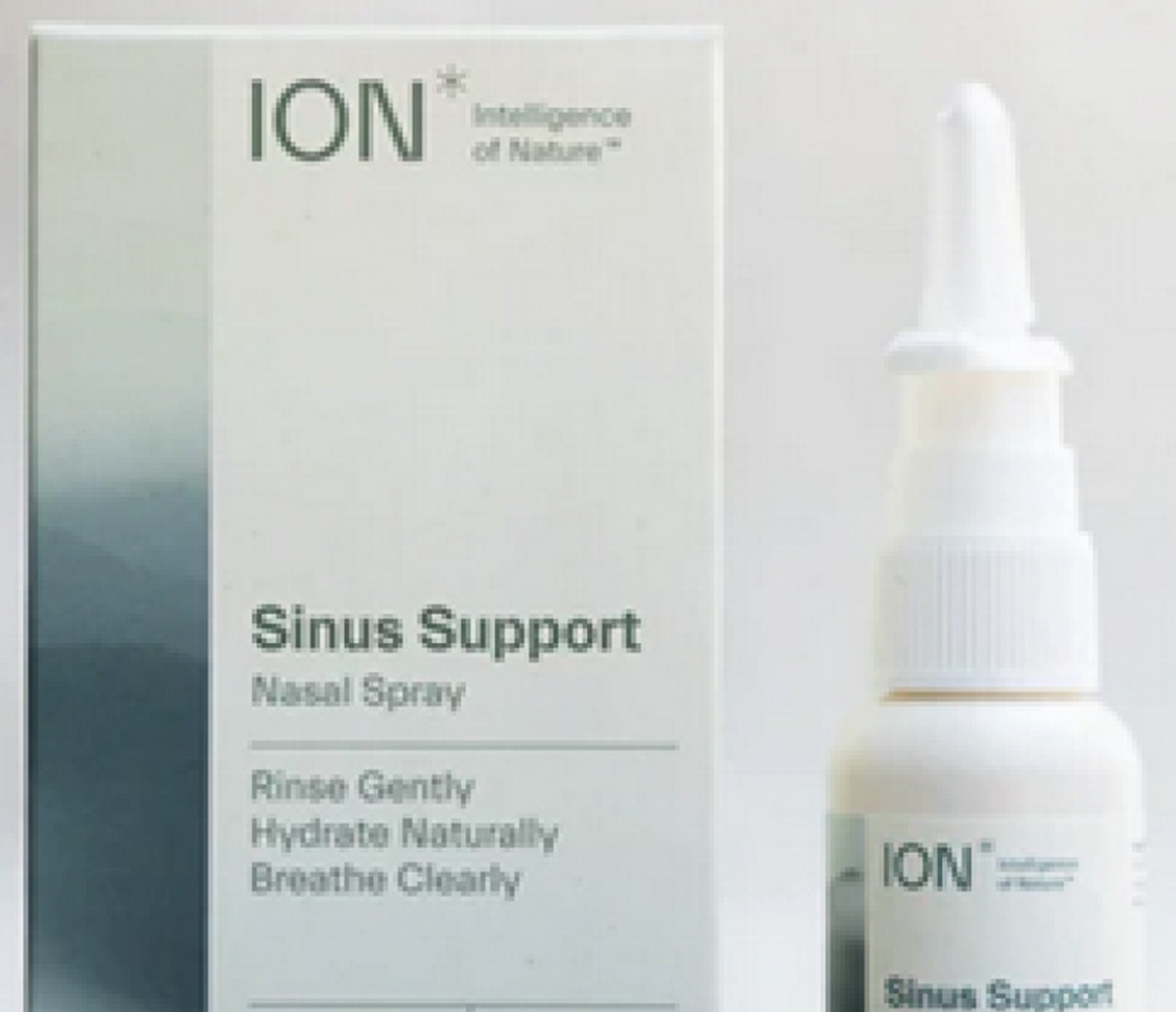
Biomic Sciences has announced the recall of all lots of ION Sinus Support, ION Biome Sinus and Restore Sinus Spray after Food and Drug Administration testing found microbial contamination in the products.
“In the population most at risk, patients or individuals who recently underwent nasal or sinus surgery, there is a reasonable probability that the use of the affected product could potentially result in severe or life-threatening adverse events such as bacteremia or fungemia, invasive bacterial or fungal rhinosinusitis, or disseminated fungal infection,” FDA said in a statement.
There have been no reports of adverse events related to the product, the FDA said.
What’s included in the recall?
The product is used as a nasal rinse and is packaged in individual boxes of one or two nasal spray dispensers. All lots of the following products are included in the recall:
- ION Sinus Support sold September 2021 through Sept. 20
- ION Biome Sinus sold September 2019 through September 2021
- Restore Sinus Spray June 2017 through September 2019
The products were sold in both wholesale and retail outlets. People who have the products are advised to stop using them and discard them. Customers who take a photo of the lot number on the bottom of the bottle before throwing it away can receive a refund. You can get more information at 1-844-715-0113.