Stop using these eyedrops sold at CVS, Walmart, FDA warns
The Food and Drug Administration has recalled eye drops sold at CVS and Walmart over concerns of “lack of sterility assurance,” following an inspection at the manufacturer’s facilities in India.
Manufacturer Brassica Pharma Pvt. Ltd is voluntarily recalling eye ointment products with expiration dates from February 2024 to September 2025.
“For those patients who use these products, there is a potential risk of eye infections or related harm. These products are intended to be sterile. Ophthalmic drug products pose a potential heightened risk of harm to users because drugs applied to the eyes bypass some of the body’s natural defenses,” the FDA announcement said.
To date, Brassica has not received any reports of adverse reactions from the products.
The products were sold nationwide via Walmart, CVS and AACE Pharmaceuticals.
The recalled products include:
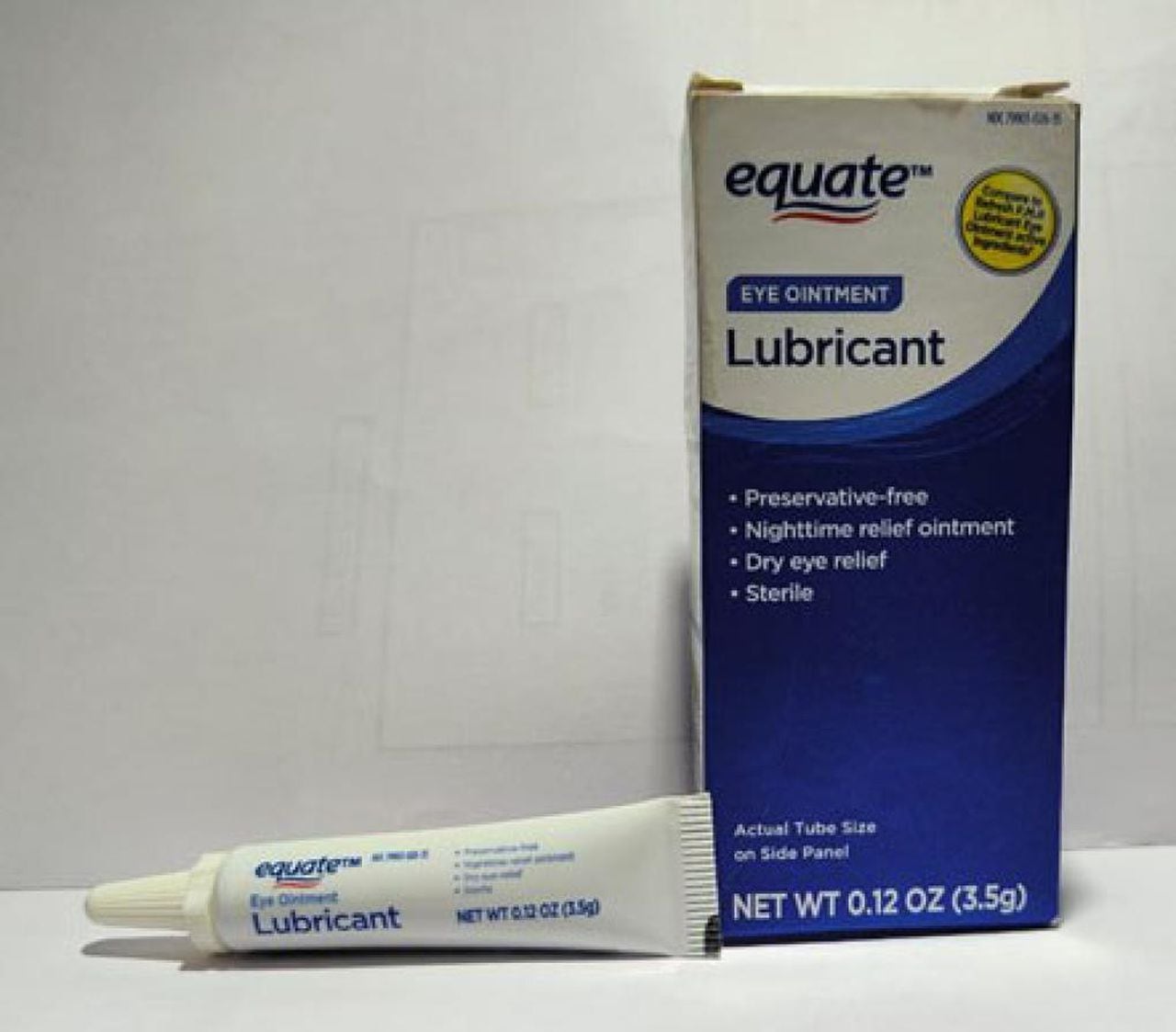
- Equate Lubricant Eye Ointment – 3.5 gram tube with UPC Code 681131395298
- Equate Style Lubricant Eye Ointment – 3.5 gram tube with UPC 681131395304
- CVS Health Lubricant Eye Ointment – 3.5 gram tube with UPC 050428634141
- Lubricant PM Ointment – 3.5 gram tube with UPC 371406124356
You can go here to see the complete product lists.
Consumers who bought the products should start using them and return them to the place of purchase.
Equate Eye Ointment LubricantCourtesy FDA
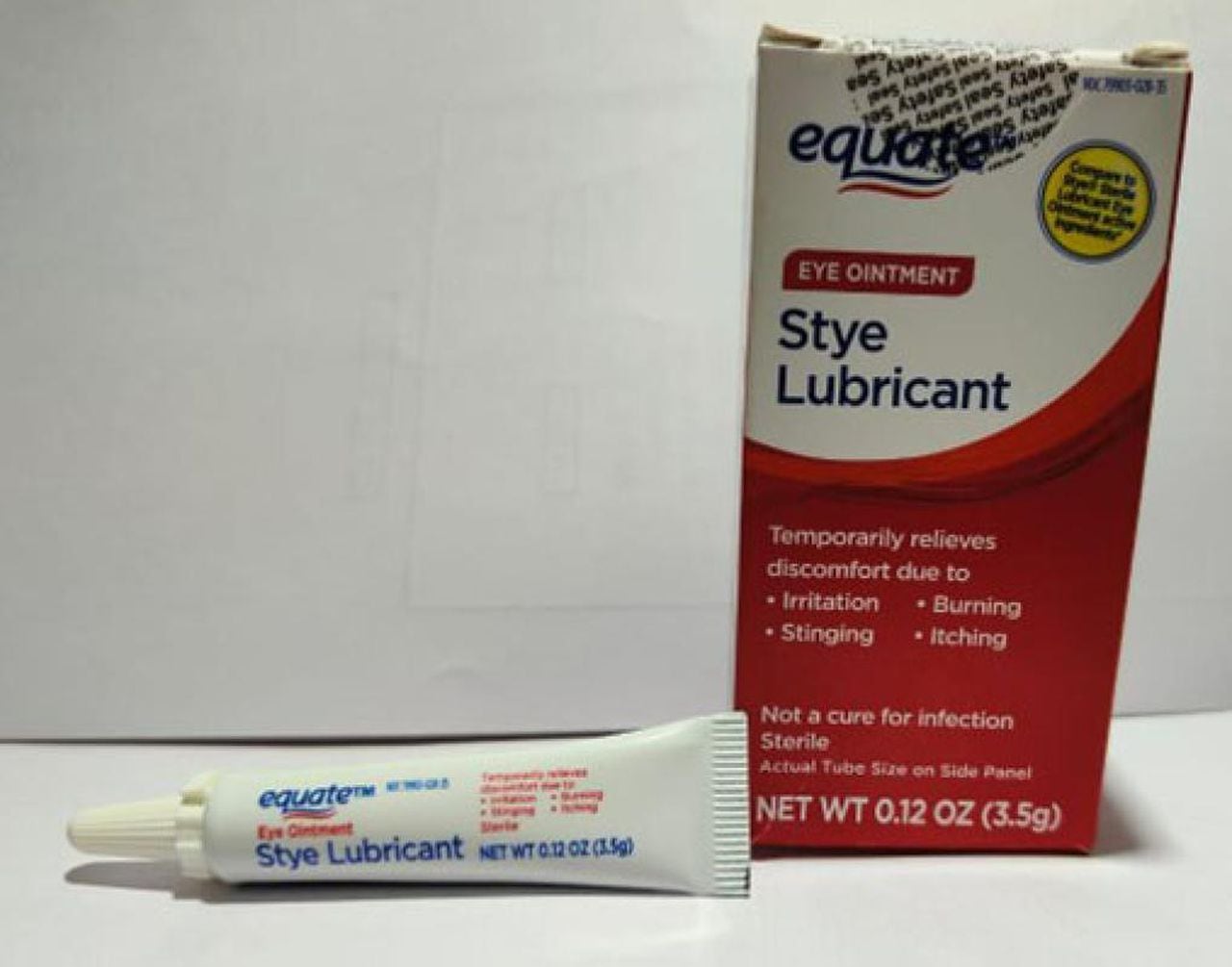
Equate Eye Ointment Style LubricantCourtesy FDA