Stop using these eyedrops: List of recalled eyedrops due to possible bacterial contamination
The Centers for Disease Control and Prevention and the Food and Drug Administration is continuing to investigate a multi-state outbreak of a drug-resistant strain of bacteria that has led to the recall of three eye drop brands.
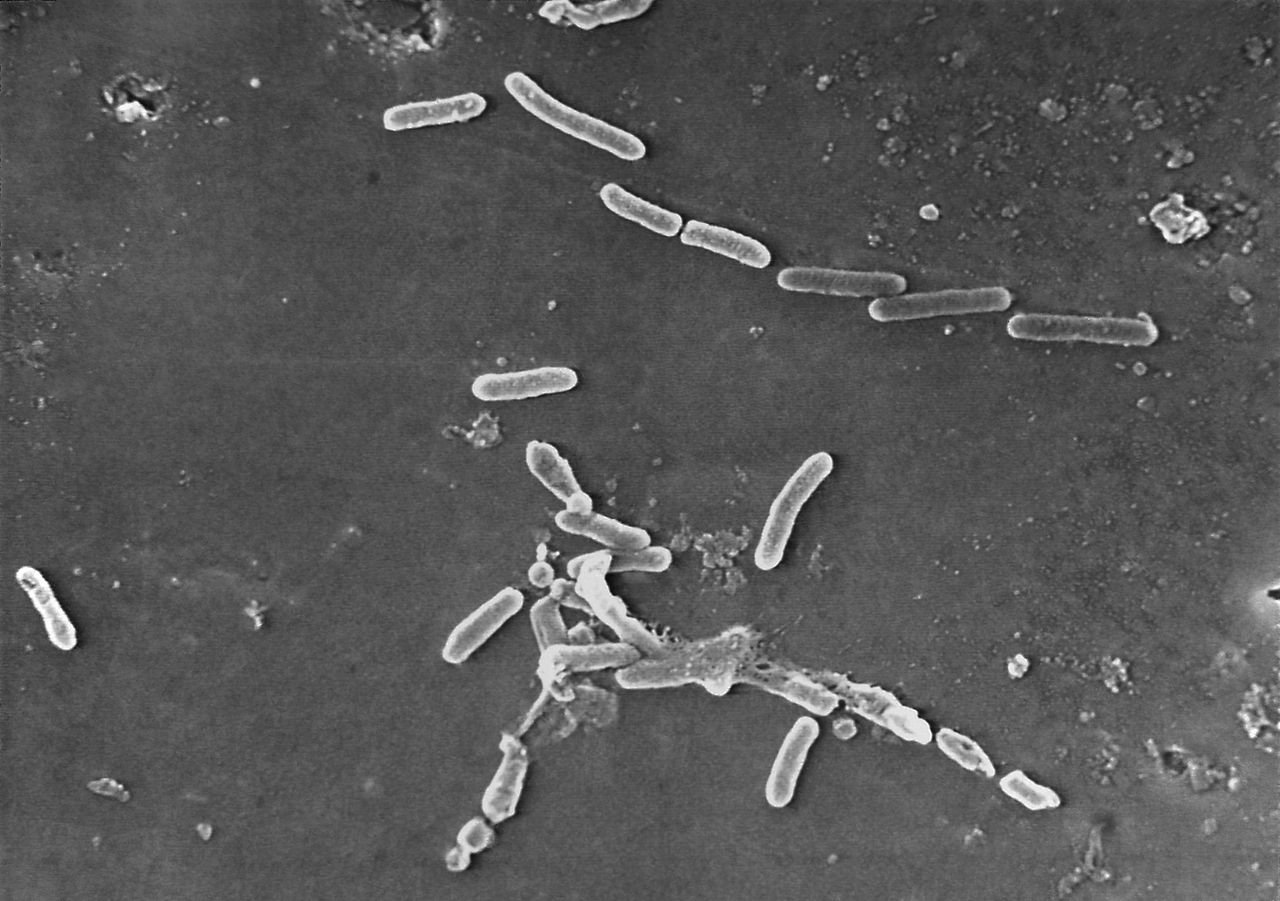
According to the CDC, there have been 58 cases in 13 states of a rare strain of drug-resistant Pseudomonas aeruginosa, a bacteria never reported in the U.S. prior to this outbreak.
The infections have led to one death and five reports of vision loss.
No cases have been reported in Alabama.
Most of the patients reported using artificial tears and one, EzriCare Artificial Tears, a preservative-free, over-the-county product packaged in multidose bottles, was the brand most commonly reported.
The initial recall included Ezri Care Artificial Tears Lubricant Eye Drops and Desam Pharma Artificial Tears Lubricant Eye Drops. It was later expanded to include Delsam Pharma Artificial Eye Ointment. All of the products are manufactured by Global Pharma Healthcare. You can go here to see a list of more product information.
Patients who have any of these eye drops are advised to stop using them immediately. Those who have used the drops or ointment and have symptoms of an eye infection should immediately seek medical care.
Eye infection systems include:
- Yellow, green, or clear discharge from the eye
- Eye pain or discomfort
- Redness of the eye or eyelid
- Feeling of something in your eye (foreign body sensation)
- Increased sensitivity to light
- Blurry vision
The FDA said the recall comes due to the failure by the company to adequately test its product for bacterial contamination without adequate preservatives, a violation of the Good Manufacturing Practice regulations that requires sterile products.