Stop using these eye drops immediately, federal officials say as another product recalled
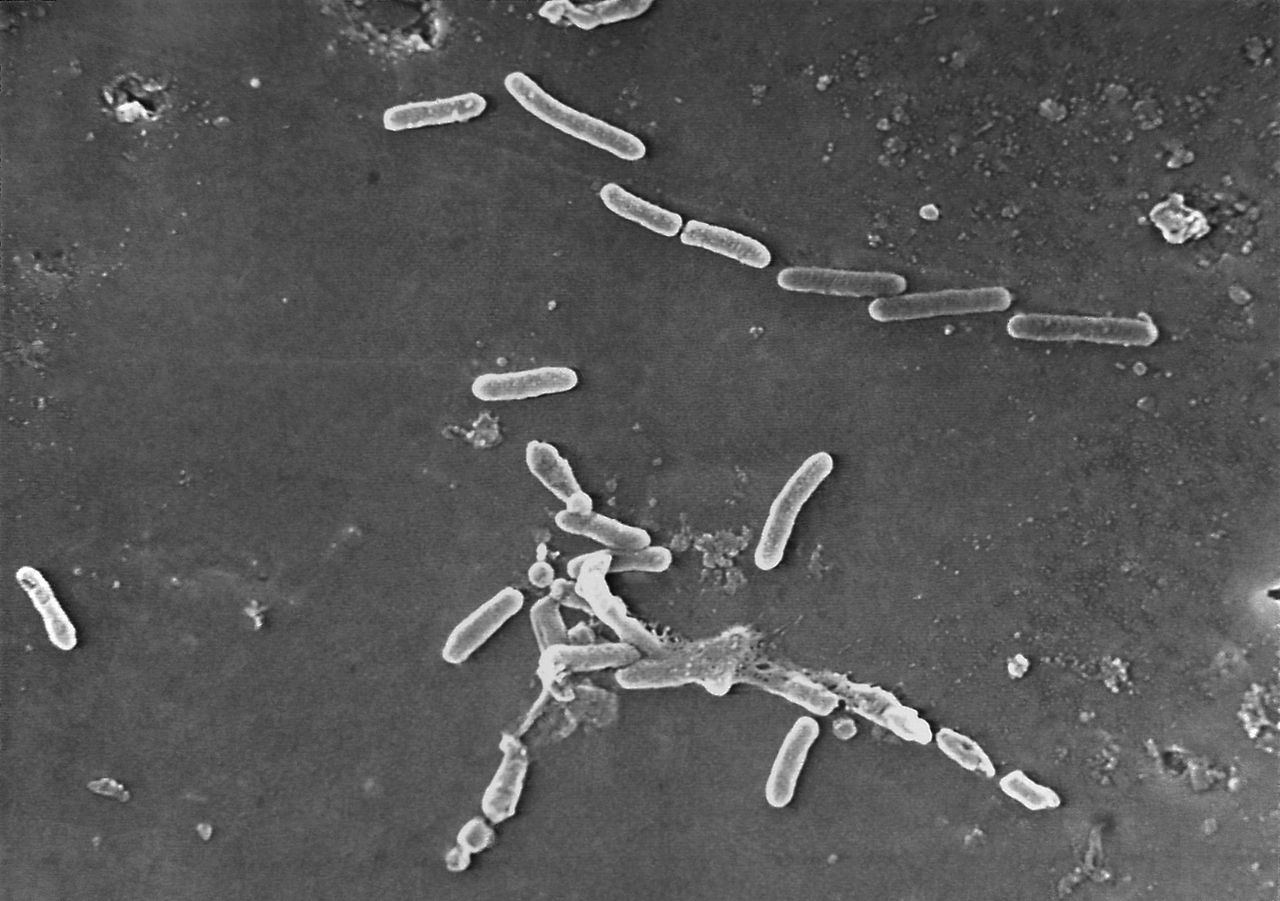
A recall for eye drops has been expanded over concerns they could be contaminated with a dangerous bacteria.
Maker Global Pharma Healthcare Private Ltd. has initiated a recall for Delsam Pharma’s Artificial Eye Ointment, according to a Wednesday announcement from the Food and Drug Administration. It’s one of a growing number of recalls linked to an outbreak of drug-resistant infections that have led to vision loss, blindness and death.
READ MORE: Eyedrop recall: Artificial tears linked to loss of vision, 1 death
The FDA had previously issued warnings about EzriCare Artificial Tears and Delsam Pharma’s Artificial Tears over contamination concerns. Both are manufactured by Global Pharma Healthcare.
According to the Centers for Disease Control and Prevention, 58 patients in 13 states have reported infections linked to EzriCare’s drops. Alabama is not included among the 12 states.
The drops have been tied to hospitalizations, with one death from a bloodstream infection, and permanent loss of vision following eye infections.
In its notice, the FDA said it recommended the initial recall due to India-based Global Pharma’s violations of good manufacturing practices, including lack of appropriate microbial testing, formulation issues, and lack of proper controls concerning tamper-evident packaging.
Delsam Pharma’s Artificial Eye Ointment is sold over-the-counter. Anyone who has it should discontinue its use and seek medical attention for any eye infections.