Eyedrop recall: Artificial tears linked to loss of vision, 1 death
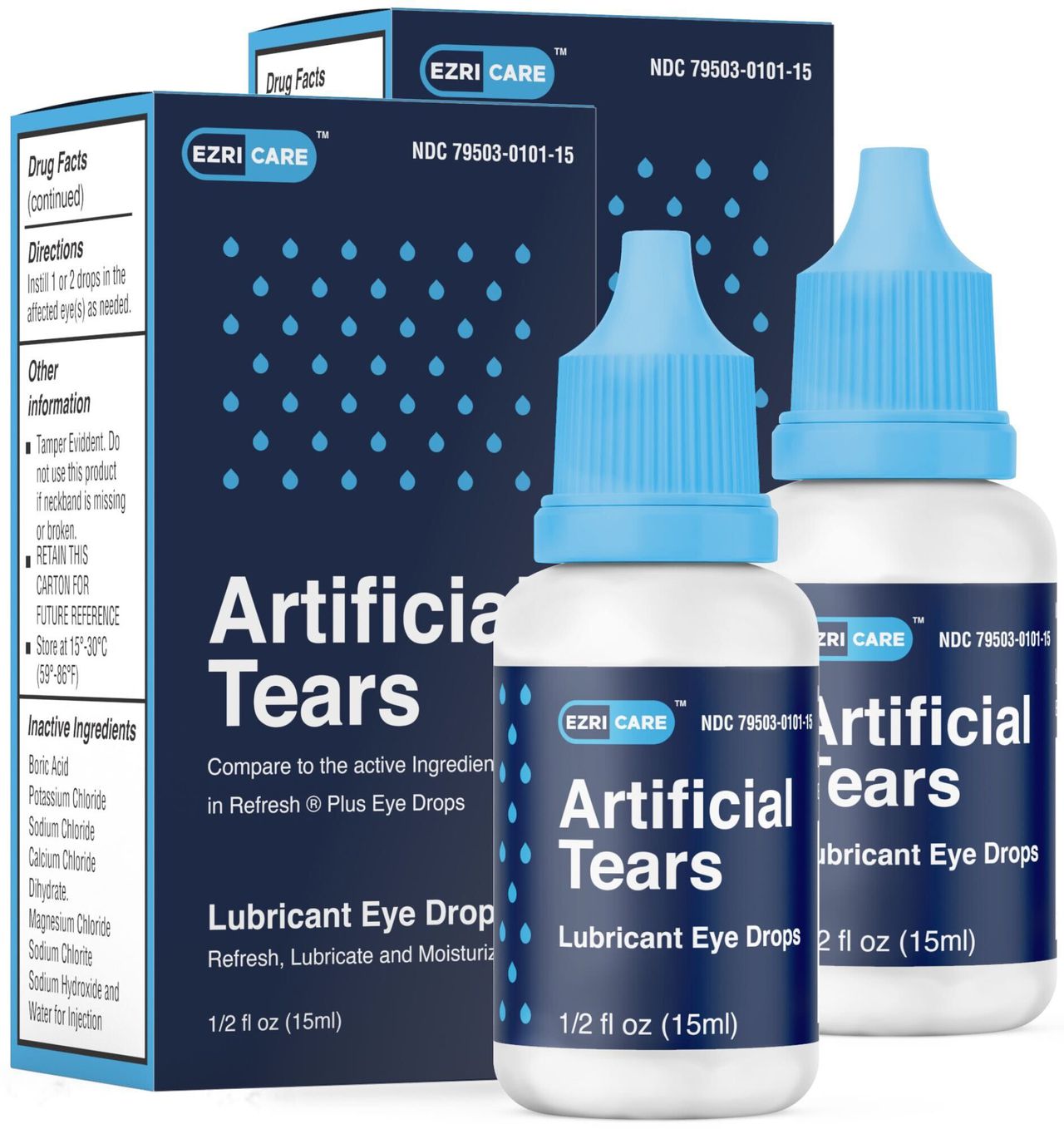
Eyedrops sold nationwide under the brand name ExriCare have been recalled due to possible bacterial contamination, the Food and Drug Administration announced.
According to the FDA, India-based Global Pharma Healthcare is recalling the Artificial Tears Lubricant Eye Drops, distributed by EzriCare, LLC- and Delsam Pharma, after they were linked to at least 55 cases of bacterial infection in 12 states. Five of those people experienced vision lost; one died when the bacteria – Pseudomonas aeruginosa, a bacterium resistant to most antibiotics – entered their blood stream.
The Centers for Disease Control and Prevention had previously urged people to immediately stop using the drops. Patients who used EzriCare artificial tears who have symptoms of an eye infection, including things such as eye pain or discomfort, yellowish discharge from the eye, eye redness and increased sensitivity to light, should seek immediate medical care.
The products were sold nationwide via the internet. Consumers with questions regarding this recall can contact the distributors: Aru Pharma/Ezricare, LLC – by phone: 1-518-738-7602 or by e-mail: [email protected].